Aluminium oxide has many applications in which its properties of extraordinary hardness and thermal stability are exploited. Examples of products incorporating aluminium oxide include abrasive materials, bone substitutes, melting pots and watch glasses. Aluminium oxide is a classic antacid remedy for heartburn.
How can I come into contact with this material?

sanding paper © Olivier-Le-Moal / fotolia.com
When used as an abrasive material in sand paper (in which it is often described as corundum), inhalable aluminium oxide dust is generate. Therefore it is recommended to take appropriate protective measures like wearing of breathing masks or installation of suction exhaust pipes in cases where high level usage occurs in confined spaces. However aluminium oxide nanoparticles tend to clump together in (humid) air resulting in lower amounts of released inhalable dust. For medical purposes aluminium oxide is taken in orally in form of tablets as a treatment for heartburn. Aluminium oxide is tightly bound in products such as watch glasses, pots and other ceramics and there is very little possibility for aluminium oxide nanoparticles to enter the human body from these products.
Is there any risk from this material to humans and the environment?
Aluminium oxides rank amongst the less toxic substances and only exhibit toxic effects in high concentrations. Inhalation of aluminium oxide dust should be avoided, but there is no evidence of significant harm to the lungs associated with the inhalation of aluminium oxide dust. Oral intake (swallowing) of aluminium oxide nanoparticles like in tablets used to treat the symptoms of heartburn is considered to be non-toxic. However, the oral intake of aluminium oxide over a long time period should be avoided as elevated aluminium levels in the blood could cause side effects on human health.
Conclusion
In our everyday life the human body is only exposed to very small amounts of aluminium oxide nanoparticles and there is no known danger associated with this material for human health.
By the way…
- Pure aluminium oxide nanoparticles cannot pass the blood-brain barrier.
- Dissolved aluminium salts do not increase the risk of contracting Alzheimer’s disease
Properties and Usage
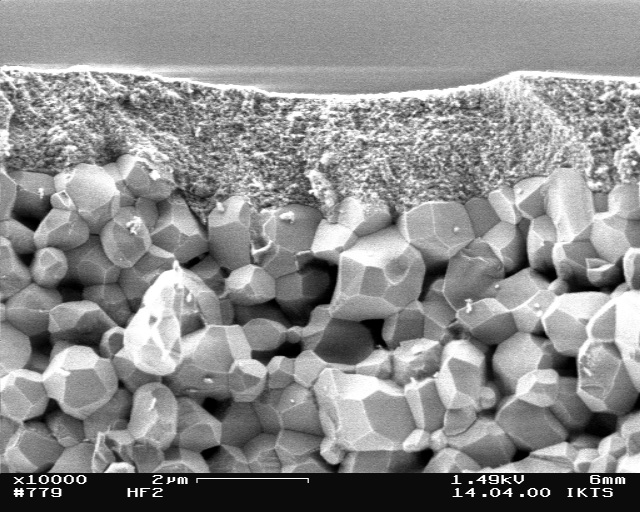
The image shows a 0,2 µm cover membrane of amorphous Al2O3 on an approximately 1 µm intermediate layer of gamma-Al2O3 on a coarser corumdum (alpha-Al2O3) substrate. © Dr. Andreas Krell, Fh-IKTS
Compounds of aluminum and oxygen are referred to as aluminum oxides (Al2O3), whereas compounds with hydroxyl groups are known as hydroxides. Alpha-Al2O3 (corundum) is the most well-known and significant form of the existing Al2O3 modifications. Besides, there are further aluminum oxides of different structures called transition aluminas [1, 2]. Corundum has a density of 3.98 g/cm³, a high hardness, a melting point of 2053 °C, and a high specific electrical resistance of approximately 1012 ohm·m (at 20 °C) [3]. It is chemically very stable and is almost insoluble in water, acids, and bases. The transition aluminum oxide gamma-Al2O3, in contrast, dissolves in strong acids and in bases. Due to its high surface activity, gamma-aluminum oxide is used as an adsorbent and catalyst material. In addition to oxides of aluminum, there are different hydroxides, e.g. aluminum hydroxides [Al (OH)3] such as bayerite and gibbsite and the so-called aluminum oxy-hydroxides [AlO(OH)] boehmite and diaspore. Gibbsite, boehmite, and diaspore are constituents of the technically important aluminum mineral bauxite.
Due to its high hardness, corundum is used as a bearing jewel in watches and as a grinding and polishing agent for precious stones, metals, and Silica wafers. Mixed with binders, especially with other oxides, corundum powder is used for manufacturing crucibles, dishes, sheath tubes, electrical insulators, artificial joints (artificial hip or knee joints), dental ceramics, burner tubes, catalyst carriers, wear protections, hard-facings, furnace linings, and metal forming and machining tools. Corundum has a low toughness in spite of its hardness and brittleness. To obtain products with improved ductility or toughness, partially stabilized zirconium oxide or titanium carbide („black ceramics“) are added to the white corundum. The low electrical conductivity and high dielectric strength of aluminum oxide are exploited in manufacturing ignition plugs and insulators. Synthetic corundum crystals grown from corundum melts have a high hardness, transparency, and scratch resistance. The precious stones sapphire and ruby consist of corundum with certain additions of iron/titanium or chromium. Synthetically manufactured sapphires and rubies are used in lasers. Sapphires are also used as scratch-resistant watch glasses.
Due to their thermal stability, boehmite and other aluminum oxides are used as catalyst carriers and adsorbents in the petroleum and chemical industries. Sintered into porous structures and applied to coarser substrates, nanoscale aluminum oxide can also be used for nanofiltration. Aluminum hydroxide in powder form is used as a flame retardant and as filler in carpets, rubbers, plastics, and foamed plastics. Moreover, it is used in dentifrices and cosmetics. Aluminumoxides and -hydroxides are often used in the dye and plastics industries as thickeners and fillers and as agents that reduce adhesiveness and increase scratch resistance. Besides, they serve to enhance the color saturation of paints and varnishes.
Alumimium oxide is not self-inflammable as nanometer-sized powder. Also as a mixture with air (dust) under the influence of an ignition source, alumina is not inflammable, so there is no possibility of a dust explosion.

Small additions of foreign substances can make sintered corundum exhibit a broad spectrum of colors. © Dr. Andreas Krell, Fraunhofer IKTS
Further applications comprise:
- ceramics: to ensure high abrasion and fire resistance
- additives for paper manufacture: to avoid that the paper adheres to the feed rolls during high-speed processes
- artificial precious stones such as sapphires or yttrium-aluminum garnets: the latter of which are used, for example, in high-energy lasers
- luminescent substances and phosphors with aluminum oxides as substrates
Occurrence and Production
Aluminum oxide is produced industrially from the mineral bauxite. The bauxite deposits are estimated at approximately 20 billion tons worldwide, the worldwide annual output amounts to about 100 million tons. Australia has the largest output and deposits. The sapphire, well-known as a precious stone, is a quite rare but at the same time the most beautiful modification of aluminum oxide. Aluminum and aluminum oxide are manufactured by means of the Bayer method: Bauxite is crushed, dried, and dissolved using concentrated sodium hydroxide solution. The impurities iron, silicon, and titanium are separated from the bauxite in the so-called red mud. Aluminum hydroxide is precipitated from the solution and calcinated at 1200-1300°C to form Al2O3.
NanoCare Data Sheets:
- Boehmite Data Sheet No.1 (PDF, only in German)
- Boehmite Data Sheet No.2 (PDF, only in German)
Further information:
- Wefers, K and Misra, C (1987). Oxides and Hydroxides of Aluminum, Alcoa Technical Paper No. 19, Alcoa Laboratories, Pittsburgh, PA, 1987.
- Petzold, A and Ulbricht, J (1991). Aluminiumoxid: Rohstoff, Werkstoff, Werkstoffkomponente, Dt. Verl. Für Grundstoffind., Leipzig, 1991. ISBN 9783342005322.
- Alfrey, AC et al. (1976), N Engl J Med, 294(4): 184-188.
Aluminium is found in the form of aluminium ions as a natural component in drinking water and other foodstuffs, especially fruit and vegetables. Aluminium oxide particles agglomerate strongly, forming larger "particle clusters". In this form they are not very toxic to cells.
General Hazards
Aluminum-containing utensils for food storage or preparation such as kitchen utensils, cans or tins, foils or tubes from which the dissolved aluminum ions pass into the food may be additional sources of exposure. Moreover, aluminum compounds can be contained in gastric-acid neutralizers, so-called antacids, and in cosmetics and are used, for example, in roll-on deodorants due to their anti-perspirant effect. Compared to the uptake via food or antacids, the uptake of aluminum via utensils for food storage or preparation and cosmetics is rather low and amounts to clearly less than the uptake quantity that is assumed not to pose any health hazard according to an updated evaluation issued by the Joint FAO/WHO Expert Committee on Food Additives (JECFA, 2006).
Occupational-health studies of specific stresses and exposure in the aluminum powder industry have shown that finest aluminum powder can cause pulmonary fibrosis under unfavorable industrial-hygiene conditions. In Germany, the resulting disease which is referred to as aluminosis has been approved as such and workers have been recompensed for the related health problems since 1943 [1,2]. The Senate Commission of the Deutsche Forschungsgemeinschaft has fixed the maximum admissible concentration (MAK-value) of aluminum oxides to amount to 1,5 mg/m3. Workers in the aluminum powder industry or welders in the automobile industry thus are required to wear suitable breathing protection.
So far, there is no scientific proof of a correlation between increased aluminum ion uptake from food including drinking water, pharmaceuticals, and cosmetics and the Alzheimer’s disease. Neither in dialysis patients nor in aluminum workers – both belonging to the groups of persons that are definitely exposed – were the Alzheimer-typical amyloid depositions in the brain observed in extremely many cases [3].
Literature
- BAuA Schriftenreihe (1994). Arbeitsmedizinische Untersuchungen zur Belastung und Beanspruchung in der aluminiumpulverherstellenden Industrie. 1. Auflage. Bremerhaven: Wirtschaftsverlag NW Verlag für neue Wissenschaft GmbH 1994, ISBN: 3-89429-551-1.
- Kraus, T et al. (2000), Int Arch Occup Environ Health, 73(1): 61-64.
- BFR Opinion Nr.33/2007 (22.07.2007): „Keine Alzheimer-Gefahr durch Aluminium aus Bedarfsgegenständen“ (PDF, ). (in German)
Studies on Living Organisms – in vivo
Within the project NanoCare, two different types of boehmite particles (primary particle sizes of 10 and 40nm) were scrutinized in in vivo studies. The experiments on rats that were made to inhale up to 28mg/m3 of the particles five days a week over a period of four weeks showed that inflammation of the lungs due to strongly agglomerated particles only occurred in the presence of the highest concentration. Moreover, enlarged macrophages and lymph node modifications were observed increasingly [1,2,3]. Inflammations in the lungs occurred at inhaled particle doses of more than 1mg per lung. Similar results were obtained from instillation experiments carried out within the project. Instillation of more than 1,2mg of boehmite particles per lung in the respiratory tracts of the test animals caused damage to the lungs. In these studies, the NO(A)EL amounts to 0,6mg [1].
Literature
- NanoCare 2009, Final Scientific Report, ISBN 978-3-89746-108-6. (PDF-Document, 19 MB )
- Pauluhn, J (2009), Toxicol Sci, 109(1): 152-167.
- Pauluhn, J (2009), Toxicology, 259(3): 140-148.
Studies Outside of Organisms - in vitro
Studies of Al, Al2O3, and AlOOH (boehmite) particles have shown that these particles tend to strongly clog and form agglomerates. These agglomerates can be taken up by the cells but are always found in vesicles, which means that they do not occur freely in the cells and are practically never detected in the cell nucleus [2,3,4,6]. Studies of human lung cells have shown Al2O3 to have only small harmful effects on the cell division and cell vitality and have proved that even very high doses do not cause formation of harmful reactive oxygen species (ROS) [7].
Aluminum oxide (Al2O3) which, for example, is used in orthopedic ceramics, has also been investigated for its genotoxicity. Very high doses were observed to have only minor mutagenic effects. Aluminum oxide fibers were found to be more genotoxic than nano- or micro-scale particles [1]. Aluminium particles are more toxic than aluminum oxide particles [4]. Only very high doses of Al2O3 can decrease the function of the mitochondria (only at or above 200µg/ml, the function of the mitochondria is reduced by 15%) and can cause (programmed) cell death of part of the cells [5, 6].
Boehmite, an aluminum oxihydroxide (AlOOH), was studied within the NanoCare project. For the human lung cell line A549, a threshold concentration of at least 50µg particles per cm2 was determined. When the cells were treated with at least that concentration (LOEL) for more than 72hours, they were observed to become stressed while inflammation markers were produced. No effects were triggered by low doses of AlOOH in any of the different cell lines of different origins [2]. Experiments on the mobility of nanoparticles across cell barriers (such as the air-blood barrier in the lung) showed that boehmite does not pass through cells. The barrier function of the cells is not influenced by the particles.
Using the so-called vector model which displays some of the elementary cell effects [8], partners of the NanoCare project proved that AIOOH particles are among the low-toxicity materials. Excessive, overloading concentrations of 60-120µg particles per 106 macrophages were observed to damage the cells but did not lead to the formation of harmful reactive oxygen species (ROS) [2]. Damage due to realistic doses of Al particles is not expected.
Literature
- Tsaousi, A et al. (2010), Mutat Res, 697(1-2): 1-9.
- NanoCare 2009, Final Scientific Report, ISBN 978-3-89746-108-6. (PDF-Document, 19 MB ).
- Monteiro-Riviere, NA et al. (2010), J Appl Toxicol, 30(3): 276-285.
- Wagner, AJ et al. (2007), J Phys Chem B, 111(25): 7353-7359.
- Jeng, HA et al. (2006), J Environ Sci Health A Tox Hazard Subst Environ Eng, 41(12): 2699-2711.
- Simon-Deckers, A et al. (2008), Toxicology, 253(1-3): 137-146.
- Kim, I-S et al. (2010), J Nanosci Nanotechnol, 10(5): 3453-3458.
- Bruch, J et al. (2004), Int J Hyg Environ Health, 207(3): 203-216.
The element aluminum is found naturally in the earth's crust (about 8,1 g/kg soil), and various aluminum compounds are components of soils and rocks. For nanoscale Al2O3 particles, there are no measured values for actual environmental concentrations.
From the amount of Al2O3 particles employed in different consumer goods, concentrations of 0,0002g/l in water and 0,01g/kg predicted in soil were predicted by computer models [1]. In relation to the natural occurrence of aluminum in the soil and also to predicted environmental concentrations (PEC-value) for zinc oxide or titanium dioxide nanoparticles, these values are very low.
Literature
- Tiede, K et al. (2009), J Chromatogr A, 1216(3): 503-509.
Inhalation of very fine aluminium oxide dust may cause inflammation of the lungs. In contrast to the lungs, the skin is a good barrier against particles. Aluminium occurs as a natural component, as aluminium salt, in drinking water and other foods, especially in fruit and vegetable.
Uptake via the Lung – Inhalation
Permanent exposure to aluminum oxides can cause damage to the lung. The related disease is referred to as aluminosis and is characterised by pathological changes of the lung that can be caused by chronic exposure to aluminum fumes or dusts, i.e. aluminum oxide particles from the aluminum oxide film that forms as the pure aluminum oxidizes immediately upon contact with air. Aluminosis belongs to the group of pneumoconioses and is an occupational disease subject to compensation. Workers in the aluminum powder industry or welders in the automobile industry are required to wear suitable breathing protection. Dust formation during aluminum powder filling/refilling should be avoided and optimum ventilation/extraction by suction should be provided. The maximum admissible concentration (MAK value) of aluminum oxides must not exceed 1,5 mg/m3 (respirable fraction).
Literature
- BAuA Schriftenreihe (1994). Arbeitsmedizinische Untersuchungen zur Belastung und Beanspruchung in der aluminiumpulverherstellenden Industrie. 1. Auflage. Bremerhaven: Wirtschaftsverlag NW Verlag für neue Wissenschaft GmbH 1994, ISBN: 3-89429-551-1. (in German)
- Kraus, T et al. (2000), Int Arch Occup Environ Health, 73(1): 61-64.
- Chemikalienlexikon.de (DE): Material Safety Data Sheet Aluminium (status 2010).
Uptake via the Skin – Dermal Uptake
Aluminium (aluminum salts) is used in roll-on deodorants due to its anti-perspirant effect. Al2O3 tested according to OECD Guideline 404 "Acute Dermal Irritation/Corrosion" [3] in animal experiments does not cause irritations of the skin of animals. The maximum recommended daily aluminum ion uptake through the use of roll-ons is 7 µg, which is considered to be non-hazardous to health [2].
Literature
- Chemikalienlexikon.de (DE): Material Safety Data Sheet Aluminium (status 2010). (in German)
- BFR Opinion Nr.33/2007 (22.07.2007): „Keine Alzheimer-Gefahr durch Aluminium aus Bedarfsgegenständen“ (PDF, ).
- OECD (2015), Test No. 404: Acute Dermal Irritation/Corrosion, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, https://doi.org/10.1787/9789264242678-en.
Uptake via the Gastro-Intestinal Tract
In 1989, a tentative tolerable weekly uptake of 7 mg/kg weight was fixed for the total uptake of aluminum (ions) from food, including aluminum salts in food additives, by the Joint Expert Committee on Food Additives of the Food and Agriculture Organization of the United Nations (FAO) and the WHO (JECFA) and the Scientific Committee on Food (SCF) of the European Commission [3,4]. Intoxication sets at much higher doses: Al2O3 is toxic (LD50) upon swallowing of 5 g/kg weight (rat) [1], which means that an adult weighing 70 kg has to take up at least 350 g of Al2O3. 4 g are enough, however, to cause serious disorders such as mucous membrane irritations. Aluminum oxide is resorbed at a small rate only via the gastrointestinal tract. Aluminum compounds are secreted mainly via the kidneys [2].
Literature
- Chemikalienlexikon.de (DE): Material Safety Data Sheet Aluminium (status 2010). (in German)
- BFR Opinion Nr.33/2007 (22.07.2007): „Keine Alzheimer-Gefahr durch Aluminium aus Bedarfsgegenständen“ (PDF, ).
- Joint FAO/WHO Expert Committee on Food Additives (JECFA) (1989). Toxicological evaluation of certain food additives and contaminants. WHO Food Additives Series 24: 113-154.
- World Health Organisation (WHO) (2011). Guidelines for drinking-water quality, fourth edition. ISBN: 978 92 4 154815 1
The effect of nano-and microscale alumina was investigated in a variety of organisms, with only very high, often not environmentally relevant concentrations having a toxic effect. For boehmite, another alumina, no ecotoxicological studies are available.
Thus, the soil-dwelling model organisms mud tube worm (see picture), shrimp, earth worm and basket shells were investigated [1]. In this study, only the shrimp showed an impairment of growth and survival at very high, not environmentally relevant concentrations of nanoscale Al2O3. Regarding the uptake of alumina particles, large differences both between organisms and between nano-and microscale Al2O3 were observed.

The tube worm. © Wikipedia.de
For a nematode Al2O3 was toxic, as demonstrated by growth inhibition and reduction of reproduction [2]. Here nanoparticles acted stronger than coarser particles. Interestingly, for aluminum salt an even higher toxicity than for the nanoparticles had been detected. An earthworm exposed to Al2O3 in the ground for 4 weeks shows no increased mortality, even in very high, not environmentally relevant concentrations. The worms, however, were impaired in their propagation [3]. Nanoscale alumina has no strong antimicrobial properties. The metabolic activity of bacteria was not influenced by Al2O3 particles [4,5]. Very high particle concentrations, which are not expected to occur in the environment, caused a slight reduction in bacterial growth by an interaction with the bacterial surface [6]. In contrast, there are results that show a growth inhibition of different bacterial species also at lower particle concentrations [7,8]. Moreover, a stronger effect of the nanoparticles compared with larger particles was observed here. Nanoscale Al2O3 induces no mutagenic effects [9]. Daphnia responded to exposure to very high concentrations of Al2O3 nanoparticles with reduced mobility and increased mortality [10,11]. As shown in the figure, the water fleas internalise nanoparticles from the water in the intestine. An increased sensitivity towards nanoscale compared to microscale particles was observed [10].
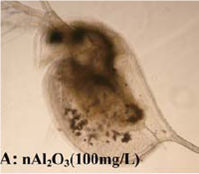
A water flea with aluminia nanoparticles in the body. © Zhu et al., 2009.
For embryos and larvae of zebrafish, however, neither nano- nor microscale particles were toxic [12,13]. Several plants, such as the California Kidney bean and ryegrass showed a normal growth in the presence of nanoparticulate Al2O3. Kidney beans internalise no particles from the soil into the leaves, while the aluminum concentration in the leaves of the ryegrass approximately doubled [14]. Also, no toxic effects of aluminum oxide on germination, root growth and leaf number of the thale cress (Arabidopsis) were observed [13]. Corn, carrots, soy, cabbage and cucumber showed a reduced root growth in the presence of Al2O3[15]. Interestingly, this effect disappeared when the particles were previously loaded with phenanthrene. It is speculated, that the phenanthrene changed certain surface properties of the particles so that they are no longer toxic. In another study, however, radish, rapeseed, rye, lettuce, corn and cucumber were not affected in germination and root growth [16]. No toxicity was observed in algae [5]. The risk posed by Al2O3 nanoparticles for environmental organisms is considered to be low. Because of little or no toxicity, often no differences between nano-and microscale particles are observed. If, however, a stronger toxic effect occurs, this is more pronounced for nanoscale than for micro-scale particles.
Literature
- Stanley, JK et al. (2010), Environ Toxicol Chem, 29(2): 422-429.
- Wang, H et al. (2009), Environ Pollut, 157(4): 1171-1177.
- Coleman, JG et al. (2010), Environ Toxicol Chem, 29(7): 1575-1580.
- Doshi, R et al. (2008), Environ Res, 106(3): 296-303.
- Velzeboer, I et al. (2008), Environ Toxicol Chem, 27(9): 1942-1947.
- Sadiq, IM et al. (2009), Nanomedicine, 5(3): 282-286.
- Jiang, W et al. (2009), Environ Pollut, 157(5): 1619-1625.
- Hu, X et al. (2009), Sci Total Environ, 407(8): 3070-3072.
- Pan, X et al. (2010), Chemosphere, 79(1): 113-116.
- Zhu, X et al. (2008), J Nanopart Res, 11(1): 67-75.
- Griffitt, RJ et al. (2008), Environ Toxicol Chem, 27(9): 1972-1978.
- Zhu, X et al. (2008), J Environ Sci Health A Tox Hazard Subst Environ Eng, 43(3): 278-284.
- Harper, S et al. (2008), J Exp Nanosci, 3(3): 195-206.
- Lee, CW et al. (2010), Environ Toxicol Chem, 29(3): 669-675.
- Yang, L et al. (2005), Toxicol Lett, 158(2): 122-132.
- Lin, D et al. (2007), Environ Pollut, 150(2): 243-250.
Both aluminium particles and aluminium oxide particles are incorporated into cells. If they are not stabilised with additives, they tend to agglomerate.
Behaviour at the Blood-Brain Barrier
Animal experiments on mice and rats were carried out to investigate the permeability of the blood-brain barrier. Very high doses of Aluminium particles were administered via the carotid artery, into the veins, the abdomen, and the hollow spaces of the brain of the test animals [1]. Since the experimental doses cannot be compared with the realistic doses, the results must be judged with caution. So far, relevant Aluminium nanoparticle doses have not been proven to cause neurotoxicity [2,3].
Literature
- Sharma, HS et al. (2009), J Nanosci Nanotechnol, 9(8): 5055-5072.
- Oberdoerster, G et al. (2009), J Nanosci Nanotechnol, 9(8): 4996-5007.
- BFR Opinion Nr.33/2007 (22.07.2007): „Keine Alzheimer-Gefahr durch Aluminium aus Bedarfsgegenständen“ (PDF, ).
Behaviour of Uptake in somatic cells
The agglomerates, which can also be taken up in the cells, are found in vesicles, i.e. they do not occur freely in the cells and are practically never detected in the cell nucleus. The agglomerated particles can be detected by means of electron microscopy in the cell inclusions. The vesicle membrane protects the remaining cell components from the particles [1,2,3,4].
Literature
- NanoCare 2009, Final Scientific Report, ISBN 978-3-89746-108-6. (PDF-Document, 19 MB ).
- Monteiro-Riviere, NA et al. (2010), J Appl Toxicol, 30(3): 276-285.
- Wagner, AJ et al. (2007), J Phys Chem B, 111(25): 7353-7359.
- Simon-Deckers, A et al. (2008), Toxicology, 253(1-3): 137-146.
Few studies have examined the behavior of engineered aluminum nanoparticles in the environment. Generally, the solubility of these particles is considered to be low, i.e. only a few ions dilute from the particles in aqueous solution [1]. Under acidic conditions, however, increases the solubility, and more ions get into soil or water. Ions were described as toxic to the roots of crops.
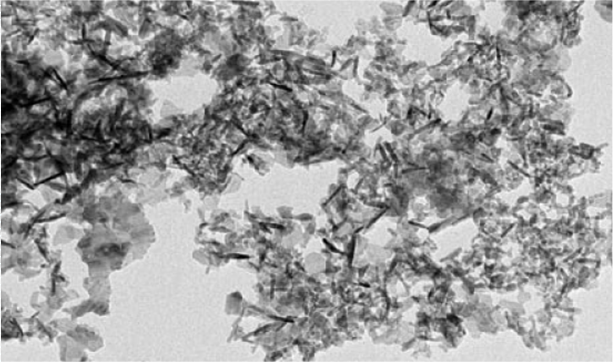
Image of aluminium oxide nanoparticles taken by transmission electron microscopy (TEM). © Stanley et al. 2010
In soils aluminum particles are mobile, but at low, acidic pH values the mobility is significantly higher [2]. In the presence of natural organic material more particles remain in the solution, i.e. the organic material influences the stability of a suspension of Al2O3[1,3,4].
Studies on the binding behaviour of various chemicals by nanoscale Al2O3 showed that different amounts were bound and toxicity of these chemicals is thus rather decreased [5]. At the same time, the binding of organic material from soil or water to Al2O3 particles increases the amount of bound chemicals (such as phenanthrene), compared with organic material alone or particles alone [6]. The effects of particle-bound chemicals on environmental organisms are not yet fully understood. However, note that the binding of organic matter on natural aluminum particles also occurs
.
Literature
- Stanley, JK et al. (2010), Environ Toxicol Chem, 29(2): 422-429.
- Doshi, R et al. (2008), Environ Res, 106(3): 296-303.
- Davis, JA et al. (1981), Environ Sci Technol, 15(10): 1223-1229.
- Ghosh, S et al. (2008), Langmuir, 24(21): 12385-12391.
- Ra, JS et al. (2008), Environ Int, 34(2): 184-192.
- Yang, K et al. (2010), Environ Sci Pollut Res Int, 17(2): 410-419.
 >
>