Silver is a noble metal that has been used in jewellery and cutlery for hundreds of years. In the electronic industry it is used for its high electrical conductance capacity and in the medical industry, the antibacterial activity of silver is utilized in wound plasters and bandages, implants, creams and lotions. Nano-scaled silver is more effective compared to coarse silver as it has a higher surface area and can be more easily distributed in the various media. The clothing industry is using silver in textiles for its anti-bacterial properties and when applied into plastic packaging materials it helps to keep the food fresh for longer periods of time. Other applications of the noble metal silver are paints, cement and carpets.
How can I come into contact with this material?

silver cutlery © ninell / fotolia.com
Only small amounts of silver are released from silver cutlery and taken up through the food. The wearing of silver jewellery presents no reason for any health concern as normal healthy skin is a good barrier preventing any uptake. Silver inhibits effectively the growth of bacteria making it an excellent ingredient for medical applications. It supports the healing process of the skin e.g. in case of wound dressings for burns or creams used to treat certain skin conditions such as neurodermatitis or skin eczema. It has been shown that silver particles can be taken up by the skin via this route. The usefulness of silver in clothing items (e.g. socks) is controversial. A major question is whether it makes sense to invest the limited silver reserves in low-odour socks since it has been shown that silver can be washed out from these textiles and will end up in our wastewater treatment plants.
Silver is also approved as a food colouring agent (known as E174) to colour product surfaces. Labelling of food items is mandatory in cases of sugar-, confectionary and bakery products. Plastic packaging materials sold in the US or in Asia could contain silver particles which may then change over to the covered food items and thus enter the human body. Silver included in spray applications can also be breathed in.
Is there any risk from this material to humans and the environment?
Silver has bactericidal properties and if silver nanoparticles are administered in small amounts they will not cause any harm to the human body. However, the use of silver in high amounts and over a long period can cause a grey colouring of the skin and organs. It is important to notice that silver is especially toxic to certain wildlife animals such as fish.
Since the production volumes and applications of silver nanoparticles are constantly increasing greater quantities of silver nanoparticles enter the environment ending up for example in sewage sludge of wastewater treatment facilities. In general metals such as silver cannot be degraded in the environment.
Conclusion
Small amounts of silver nanoparticles are non-hazardous for humans. Only high concentrations of silver nanoparticles could be expected to cause adverse health effects in the human body. At present there is no evidence of risk to the environment but the hazard potential for the environment is likely to be higher as some animal species, notably fish are especially sensitive to silver. However, the German Environmental Agency (UBA) recommends adjusting the upper limit for silver content of sewage sludge upon which it is not allowed to be used on cultivated fields. Some countries, e.g. Switzerland, already have installed a legal ban for the usage of sewage sludge on cultivated fields.
By the way…
The threshold limit value (TLV) for silver (in silver-containing compounds) allowed to be inhaled (breathed in) at the workplace is 0,01 mg/m3.
Properties and Applications
Silver (chemical symbol Ag) is one of the best-known metals. Be it the silver cutlery in family possession for over 100 years or silver jewellery that slumbers in a treasure chest. The “silvery”-white metal has been known and appreciated since ancient times. Because it is more common (about 20 times) and because of its lower chemical stability it is not as expensive as gold. The lower chemical stability is illustrated by the fact that silver tarnishes – in most cases this means that silver reacts with sulphur compounds e.g. from perspiration to black silver sulphide. In contrast, gold is so precious that it hardly reacts with “normal” reagents and therefore does not tarnish.
For the chemical industry silver plays only a minor role e.g. as a catalyst. A classical area of application was the traditional photography. Photographic film contained silver salts that were reduced to elemental silver through exposure, thus producing the desired photo. Film developing in many photo labs lead to unnecessary silver being washed out and ending up in sewage treatment plants (keeping in mind the discussion whether nanosilver is damaging to the sewage treatment plants). Since the digital camera has reached private and professional households, the contamination of sewage water with silver has to have reduced; the classical (silver) film is today an “endangered species”.
Another important area of application for silver is the electronics industry, as silver has one of the highest conductivities for electrical current and is therefore very important for the electronics industry. Combined with great ductility and the chemical stability described above, silver is a highly demanded material for electronic devices or circuits. Brazing alloys containing silver and other metals are used for brazing joints.
Silver can play a role in biological systems as well. Already the old Romans knew about the bacteria inhibiting property of silver, they put silver coins in jars that were used for the storage of milk. The minimal release of silver and thus the release of silver ions lead to a longer preservation of the milk. Because the bactericide (biocidal) effect was only weak, it was possible to drink the dissolved silver without any problems.
For the treatment of warts, which are mostly caused by viruses, silver nitrate is used in the form of a solid stick, the so-called lunar caustic The effect of this stick is commonly described as cauterization, this means the silver nitrate releases corrosive nitric acid when exposed to light and this etches off the wart. At light, the remaining silver ion is reduced to elemental silver that causes a blackening of the treated skin area. Because the reduction of (atomic) silver ions produces black silver particles ranging in size in the upper nanometer or micrometer scope, there have to exist silver particles with dimensions of a few nanometers at some point in time in between. According to the present definition, the traditional lunar caustic performs a kind of nanotechnology.
first-aid box © PeJo / fotolia.com
In medicine (nano) silver is used as a wound dressing, often the term „colloidal“ silver is used – it was called that way before the term nano was in fashion (collides can range in size between nanometers and micrometers). Nanosilver has a better effect compared to coarse silver particles, because it has a larger surface and can be dispersed more finely. Through the larger surface more silver ions are released, that finally have the bactericide (biocidal) effect. Additionally, less of the expensive silver is needed.
Because of its bactericide (etc.) effects silver is also used in other products. Often it is not clear whether the attribute “nano” is only used for advertising purposes or if nanoparticles were actually used – that is to say the term nano is not protected.
Furthermore, it can be argued if it makes sense to invest our limited silver supplies in low-odour socks. It is proven that silver is washed off from such textiles and ends up in sewage treatment plants (cf. the above paragraph about photography).
Silver is not self-inflammable as nanometer-sized powder. Also as a mixture with air (dust) under the influence of an ignition source, silver is not inflammable, so there is no possibility of a dust explosion.
Occurrence and Production
Silver is often mined as metal (called “solid silver”) more seldom in the form of silver salt. It is usually mixed with copper or lead and/or other metals; this is referred to as “association”.
Further Information
- Roempp Chemie-Lexikon (1992). Band 5, Pi-S, Silber, 9. Auflage, Thieme-Verlag, Stuttgart, ISBN 3-13-735010-7
- PEN - Project on Emerging Nanotechnologies (EN): Nanotechnology Consumer Products (last access date: Apr 2010)
- Fries, René, Sabine Gressler, Myrtill Simkó, André Gazsó, Ulrich Fiedeler, and Michael Nentwich. "Nanosilver (Nanotrust Dossier No. 010en - November 2010)." In ISSN: 1998-7293, 6. Wien, 2010. https://doi.org/10.1553/ITA-nt-010en.
Studies with various consumer products show a possible release of nano-silver depending on the application and processing of the silver nanoparticles in the product. Studies show that silver nanoparticles administered in low doses do not cause any health effects, although silver can be detected in organs regardless of the respective uptake route.
General Hazards
Regardless of the particle size permanent exposure to silver can cause diseases, where high doses of silver are taken in and deposited in the body. Symptoms of the so-called argyria include irreversible staining of the skin and mucous membranes, whereas in case of argyrosis the silver deposits are locally confined. In Germany the occupational limit values for the handling of powdered substances are also applicable for silver nanoparticles and should not exceed a value of 0.01 mg/m³ for inhalable silver compounds .
Looking at many consumer products it is not always clear whether silver is actually added in the nano form for functional or for promotional purposes only. To create more transparency for the consumers the EU has put into place mandatory labelling for nano-containing products in the cosmetics and biocides sector since July 2013 and for food since December 2014. The normal calculated dietary intake of silver is approximately 70-90 mg per day .

Can textiles release nanoparticles? © Used with permission from Von Goetz, N et al. (2013). Migration of Ag- and TiO2-(Nano)particles from textiles into artificial sweat under physical stress: experiments and exposure modelling. Environ Sci Technol, 47(17): 9979-9987. Copyright © 2013 American Chemical Society.
In Germany nano silver is not approved for use as a food supplement and considered a pharmaceutical product (colloidal silver) subjected to the regulations of the German Medicinal Products Act. Dietary supplements containing nanoscale heavy metals such as silver, gold, platinum, palladium, and iridium, however are already sold internationally on the internet and are therefore also available to German buyers.
Commercially available products with integrated silver nanoparticles, such as performance sportswear, food packaging and various children's products have been shown to release silver ions and silver nanoparticles. Studies in which the wearing of silver nanoparticles containing sportswear was simulated in the laboratory have shown that both silver ions and silver nanoparticles are being released into artificial sweat. This would correspond to a worst case scenario of a non-negligible maximum exposure of the skin with 8.2 - 17.1 micrograms of silver per kg of body weight .
The same is also true for food packaging materials, but in this case the amounts found were far below the natural silver-load of humans. Nevertheless, in March 2014 the US Environmental Protection Agency (EPA) has banned the sale of the plastic containers investigated in this study .
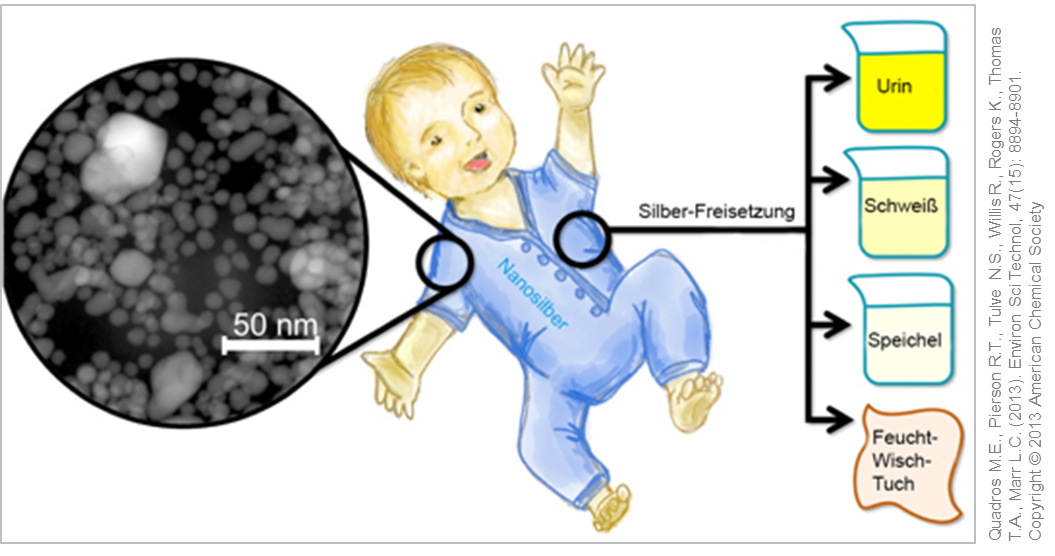
Analysis of (nano) silver containing consumer products for children. © Used with permission from Quadros M.E., Pierson R.T., Tulve N.S., Willis R., Rogers K., Thomas T.A., Marr L.C. (2013). Environ Sci Technol, 47(15): 8894-8901. Copyright © 2013 American Chemical Society
In another study, various children's products including toys, clothes and wipes were tested on a possible release of nano silver or silver ions. Here the silver nanoparticles were firmly embedded in the product, so that only very small amounts of silver ions could be detected in artificial sweat or urine .
Investigations of spray products that, according to the product description, contain silver nanoparticles only detected the silver in the form of silver chloride and silver agglomerates, but not in the nano form. However, the calculated exposure levels with silver for a worst case scenario were still below the WHO (World Health Organization) defined daily limit of 5 micrograms of silver per kg body weight .
First human studies with commercially available silver nanoparticle products, e.g. in textiles, sprays or nano silver suspensions for oral intake, showed no negative health effects of the studied subjects. Nevertheless examining the products for longer periods as well as a possible chronic exposure to silver nanoparticles are necessary steps for a comprehensive assessment of consumer products containing silver nanoparticles .
Taken together, it is not possible to make a general statement on the potential dangers of silver nanoparticle containing consumer products. Important clues are provided from the processing and integration of silver nanoparticles into the respective product.
Studies on Living Organisms – in vivo
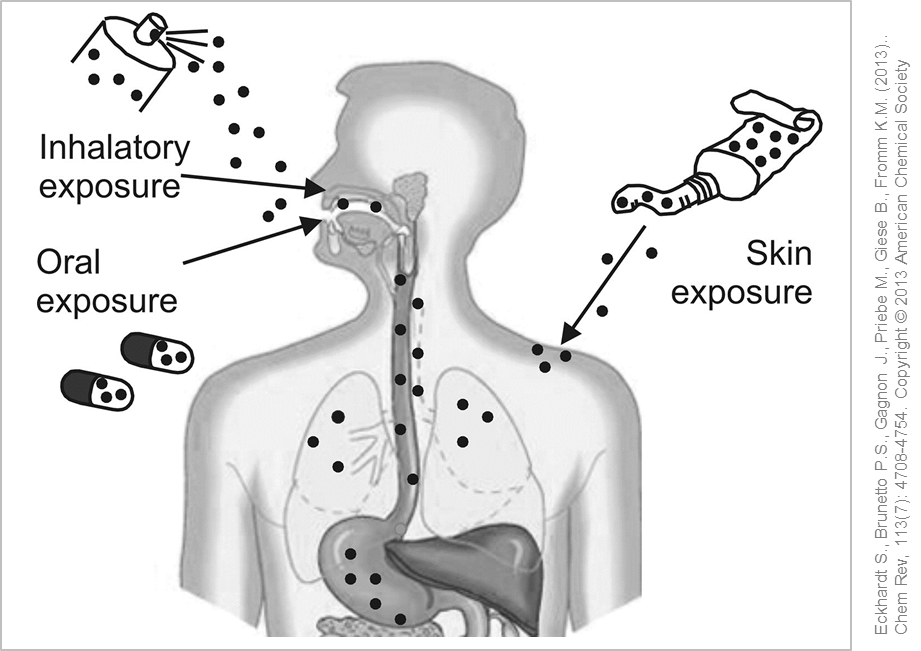
Possible entry routes of silver nanoparticles into the human body. © Used with permission from Eckhardt S., Brunetto P.S., Gagnon J., Priebe M., Giese B., Fromm K.M. (2013). Chem Rev, 113(7): 4708-4754. Copyright © 2013 American Chemical Society
To examine the possible entry routes of silver nanoparticles in the body experiments with laboratory animals were performed over longer periods of time (28-90 days). After administering silver nanoparticles to test animals via inhalation, small amounts of silver were detected in the blood and various organs such as liver, kidney, lymph nodes and brain. No definite clinical or histopathological effects were found in rats exposed to nano silver over 28 days. In the course of long-term studies over 90 days the rodents displayed decreased lung function and inflammation in the lungs .
Feeding the laboratory animals with a nano silver supplemented diet over a longer period of time also resulted in silver being detectable in the blood and different organs such as liver, lung, kidneys, stomach, testicles and in the brain. Related to this exposure scenario there occurred gender-related differences in distribution and retention of silver nanoparticles in the body. Twice as much nano silver was found in the kidneys of female rats compared to their male counterparts, whereas the male test animals showed a persistent accumulation of silver in the brain and testes. However, it is still unclear whether either silver ions or (nano) particles are absorbed via the gastrointestinal tract and transported to other areas in the body.
As silver ions are very reactive they usually agglomerate immediately upon release or interact with and bind to other chemical compounds making them more bioavailable for the body. Analyses of the genetic material revealed no DNA-damage to either male or female test animals. Repeated doses of silver nanoparticles in the diet were not toxic to the animal and had no effect on fertility of the rats .
Silver nanoparticles used in wound dressing material protect damaged skin (e.g. from skin burns) against excessive bacterial colonisation by the release of silver ions. Studies in rats have shown that the silver nanoparticles can penetrate deep into the damaged skin of rats where they work effectively against microorganisms and promote healing. Another study confirmed that the dermal application of silver nanoparticles only triggered a slight irritation of the skin but no acute toxicity .
First studies with volunteers using commercially available nano silver containing products (T-shirt, spray, silver nanoparticle solution) showed no adverse health effects on the human body. Taken together small and thus realistic amounts of silver nanoparticles are not toxic for humans and mammals based on the current scientific knowledge .
Studies Outside the Body – in vitro
Typically in the lab different cell lines are used as representative models for the respective routes of exposure under investigation (lung, skin, gastrointestinal tract) or as models for various target organs. These in vitro studies showed a dose-dependent effect of silver nanoparticles similar to the results obtained in animal experiments (in vivo studies). Besides cellular shrinkage and cell death (apoptosis) the generation of inflammation markers and the formation of reactive oxygen species (ROS) (oxidative stress) could be detected as well as further activation of stress signalling pathways within the cells .
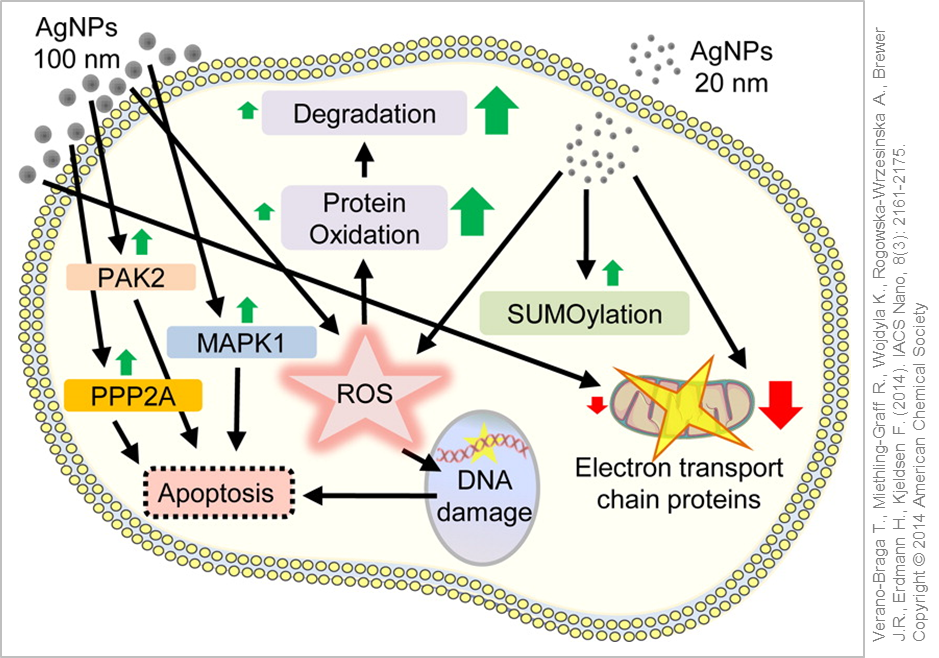
Cellular reaction and response to silver nanoparticle exposure. © Used with permission of Verano-Braga T., Miethling-Graff R., Wojdyla K., Rogowska-Wrzesinska A., Brewer J.R., Erdmann H., Kjeldsen F. (2014). ACS Nano, 8(3): 2161-2175. Copyright © 2014 American Chemical Society
It is still an open question whether it is the silver nanoparticles or rather the released silver ions or even both which are responsible for the aforementioned effects. In an aqueous environment (e.g. in in vitro experiments or in the body) you will always find dissolved silver ions together with surface-bound ionic silver in addition to the silver nanoparticles. As silver ions are very reactive and would favour the formation of ROS, they will equally interact with, and bind rapidly to, other groups (ions, sulphur groups, proteins) thus being converted into "neutral" complexes. The desired amount of released silver ions can be easily altered by specifically adapting the size and surface properties (Coatings for Nanomaterials) of the silver nanoparticles .
The majority of the in vitro studies support the "Trojan Horse" hypothesis, in which the release of silver ions only happens after intracellular uptake of the silver nanoparticles subsequently triggering the effects described (stress responses, cell death). The acidic environment within the cellular vesicles favours the dissolution of the nanoparticles. Therefore, researchers advocate to not only analyse the silver nanoparticle suspensions in in vitro experiments but also the nanoparticle-free supernatant in order to correlate the observed effects more accurately .
Depending on the type of silver nanoparticles used, some studies were able to directly link ROS formation and toxicity, whilst in other investigations ROS production and cell damage were only detectable after cellular uptake. In another analysis the silver nanoparticles activated cellular anti-oxidative protection mechanisms upon uptake thereby protecting the cell from oxidative damage. Oxidative DNA damage occurred only at very high doses which in turn further strengthened the "Trojan Horse" hypothesis .
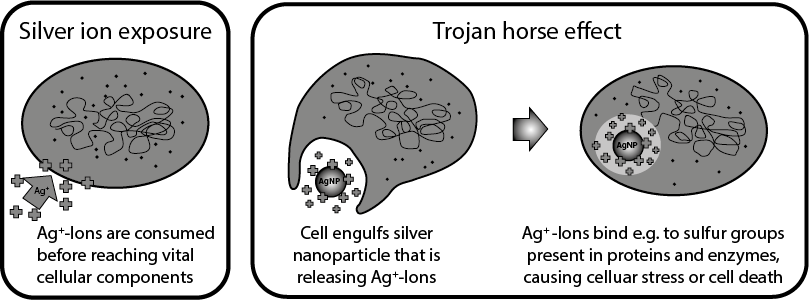
"Trojan Horse" hypothesis for the mode of action of silver nanoparticles in cells. Adapted from Quadros et al. 2011
By releasing ions, silver nanoparticles are very effective against microorganisms and therefore are often used in medicine, e.g. in wound dressings or for spray disinfectants. However, comparative studies have shown that only a relatively small therapeutic window exists for the use of silver nanoparticles in wound dressing materials, in which the nanoparticles effectively kill the undesirable pathogens and act not toxic to the cells .
The various studies have shown that relatively high concentrations of nano silver act negatively towards cellular health. As the size and surface coating of the silver nanoparticles generally have a strong influence on the nanoparticles' dissolution rate, bioavailability and distribution in the body, these properties can be altered specifically for the intended application and also to avoid undesirable side effects.
Further Information
- Arzneimittelgesetz (AMG) (2005). Gesetz über den Verkehr mit Arzneimitteln, Arzneimittelgesetz in der Fassung der Bekanntmachung vom 12. Dezember 2005 (BGBl. I S. 3394), das zuletzt durch Artikel 14 des Gesetzes vom 24. Juni 2022 (BGBl. I S. 959) geändert worden ist. (Last Access: 12/2014)
- European Union (2008). Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives (Text with EEA relevance)
Select: 1 OJ L 354, 31.12.2008, p. 16–33. http://data.europa.eu/eli/reg/2008/1333/oj (Last Access: 12/2014) - Fries, René, Sabine Gressler, Myrtill Simkó, André Gazsó, Ulrich Fiedeler, and Michael Nentwich. "Nanosilver (Nanotrust Dossier No. 010en - November 2010)." In ISSN: 1998-7293, 6. Wien, 2010. https://doi.org/10.1553/ITA-nt-010en.
- Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) (2014). EC-Report: "Are silver nanoparticles safe? Implications for health, the environment and microbial resistance."
The precious metal silver occurs naturally in the earth's crust and is present in small amounts in various environmental compartments. In the past, water bodies carried high loads of silver due to waste e.g. from the photographic industry. With the advent of digital photography, however, silver concentrations in water systems have declined drastically. Currently, it is a challenge to nanomaterials in environmental matrices. Furthermore, the natural background of silver complicates the measurement of silver nanoparticles.
 For this reason, most studies available on the occurrence of silver nanoparticles in the environment estimate or model environmental concentrations. Production volumes for silver nanoparticles are used as a basis to generate assumptions on their release from products and their subsequent distribution in the environment. The resulting predicted environmental concentrations (PEC value) can be used for risk assessment.
For this reason, most studies available on the occurrence of silver nanoparticles in the environment estimate or model environmental concentrations. Production volumes for silver nanoparticles are used as a basis to generate assumptions on their release from products and their subsequent distribution in the environment. The resulting predicted environmental concentrations (PEC value) can be used for risk assessment.
The estimated amounts of nano silver for the different environmental compartments and Europe made in 2009 and 2014 show that the estimated silver concentrations were significantly lower in 2014 despite higher production volumes. This is mainly due to an increase in knowledge about the behaviour of silver nanoparticles in the environment. The particles are subject to changes by interaction with other substances and can dissolve. By including such processes in the estimation resulted in much lower calculated concentrations .
As mentioned above, the direct measurement of nanomaterials in the environment is difficult, but it is possible to determine the total content of silver in the various environmental compartments. Comparing these measured concentrations with the predicted amounts of nano silver clearly demonstrated that the total silver amount exceeds the amount of silver nanoparticles in all considered environmental systems .
One likely release path for silver nanoparticles is the washing of silver equipped textiles. There is evidence that nano silver is released in varying amounts from textiles, and both the manufacturing and the washing process have an influence on the release behaviour. It is believed that the nano silver release from textiles and plastic products (equivalent to approximately 15 % of the total production of nano silver) is not critical for environmental organisms (see cross-cutting issues - nanoparticles in textiles) .
Up-to-now, there exist no real measurement values for silver nanoparticles for the different environmental compartments (water, soil, air). However, estimates show that nano silver represents only a small proportion of the total amount of silver in the environment. Then again, such estimated values allow only a limited evaluation of potential threats nano silver poses for environmental organisms or microbial purification stages of sewage treatment plants.
Silver nanoparticles can be absorbed into the body via the lung, skin or gastrointestinal tract and pass into the bloodstream.
Uptake via the Lung – Inhalation
Inhalation studies with laboratory animals have shown that silver nanoparticles are taken up into the bloodstream after inhalation and systemically distributed in the body. The incorporated silver could then be detected in various organs such as liver, kidney, spleen, brain and testes .
In short-term inhalation studies (28 days), no tissue damage occurred in the experimental animals exposed to sub-acute amounts of 10-15 nm silver nanoparticles, although this was accompanied by a slight inflammatory response. In the course of long-term inhalation experiments (90 days) the test animals were exposed to 18 nm silver nanoparticles for 6 hours per day via the respiratory air and showed a dose-dependent decreased lung function and inflammatory response in the lung. Even at very high doses of silver nanoparticles no genotoxic effects in the bone marrow of the animals could be detected .
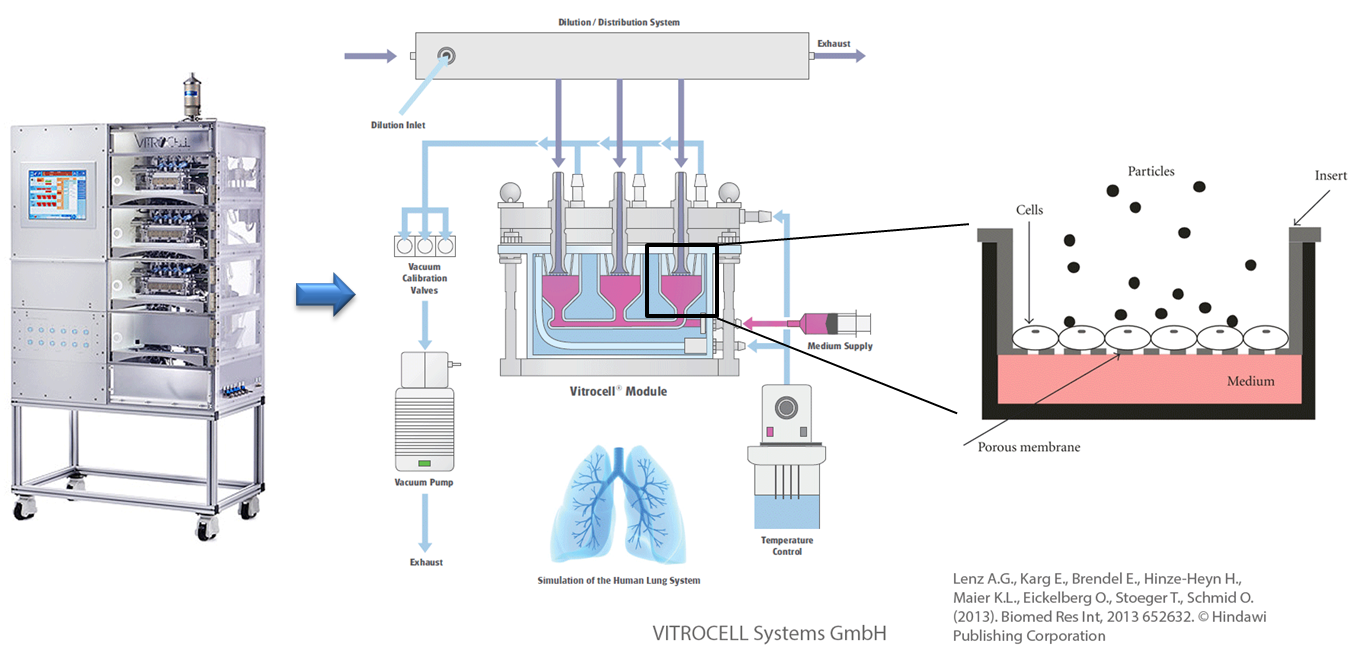
In vitro exposure at the air-liquid interface (ALI) with the example of the Karlsruhe exposure system. Used and adapted with permission from © Vitrocell Systems GmbH, Lenz A.G., Karg E., Brendel E., Hinze-Heyn H., Maier K.L., Eickelberg O., Stoeger T., Schmid O. (2013). Biomed Res Int, 2013 652632. © Hindawi Publishing Corporation
Assessing the exposure to substances at the air-liquid interface (ALI) makes it is possible to mimic and simulate the processes happening in the lung in a simplified manner, in a petri dish. Aerosolised 20 nm silver nanoparticles induced only a temporary inflammatory reaction after uptake into the cell whereas only at very high doses could adverse effects be detected. In general small silver nanoparticles trigger stronger effects in in vitro experiments as compared with larger (nano)particles due to their larger specific particle surface area .
When analysing different spray products that should contain nano silver according to the products' description, silver was only detectable in form of silver chloride and silver agglomerates, but not in nano form in the aerosol. However, even in a worst case scenario the calculated exposure levels were still below the daily WHO (World Health Organisation) exposure limits of 5 micrograms of silver per kg body weight .
In general it is possible that silver nanoparticles may enter the body via inhalation. Potential health effects however are mainly dose and time-dependent.
Uptake via the Skin – Dermal Uptake
The European Parliament has reacted to the recent fear that very small particles may penetrate the skin and exert toxic effects by changing the EU Cosmetics Directive. It stipulates that since July 2013 all nanoparticles contained in cosmetics must be labelled with the term "(nano)" as indicated on the list of ingredients.
For medical wound care, especially for skin burns, there are already several products on the market that are equipped with silver and/or silver nanoparticles. In case of silver-coated wound dressing material that was applied to damaged skin the integrated 15 nm silver nanoparticles were absorbed into the skin from this product. After one week of local treatment increased quantities of silver could be detected in the plasma and the urine of the patient without adversely affecting the health of the patient. Compared to healthy skin silver nanoparticles can penetrate much deeper into damage skin. Combining nano silver with nanocellulose results in improved wound dressing materials that were demonstrated in in vitro and in vivo experiments to exhibit a similar or even improved antimicrobial activity (compared to conventional products) combined with an accelerated wound healing and lower toxicity .
In the textile sector there are different ways to apply silver nanoparticles, with their antimicrobial effect, to the fibres. Solidly incorporated silver is not lost during washing processes allowing for a controlled and persistent long-term release of silver ions. Comparative studies with volunteers who were wearing such silver-equipped T-shirts for several weeks displayed no harmful effects to the skin or allergic reactions to the product whilst showing good antimicrobial effects. However after simulating the wearing of nano silver containing sportswear in the laboratory the studies showed that both silver ions and silver nanoparticles were being released into the artificial sweat. A worst case scenario in this simulation would correspond to a non-negligible maximal skin exposure of 8.2 - 17.1 micrograms of silver per kg body weight .
In principle silver nanoparticles can only trigger a systemic toxicity when applied to the skin if they are able to completely penetrate the skin barrier. Various studies in the laboratory (in vitro and in vivo) have tested the penetration potential of silver nanoparticles and have shown that if detectable at all, only a very small proportion of silver ions can pass through the corneal layer (stratum corneum) of the skin. After applying creams and lotions to the skin with an active-agent content (silver) in the range of 0,1 to 1,5 %, less than 0,1 percent of the amount applied was recovered in the in the corneal layer without having penetrated the skin .
If we now perform a conservative risk assessment based on these data it follows that 10 g cream with a microsilver content of 1 % contain 100 mg silver providing an estimated amount of 10 to 500 μg silver ions. At most 1 % of these silver ions are able to penetrate the deeper layers of the skin which corresponds to 0,1 to 5 μg silver ions. In total, this means in case of a generous estimate for a full body treatment (30 g of cream) 0,3 to 15 μg silver is taken up via the skin .
Uptake via the Gastro-Intestinal Tract

Food colouring agent silver (E174) used in edible food varnish. © Lottmann PR.
In a short-term study (14 days) with a commercially available nano silver product the volunteers orally received either a one-time large dose (480 g/day) or repeated smaller doses (100 micrograms/day) of silver nanoparticles. Overall the silver nanoparticles did not cause any clinically relevant negative effects in the test subjects .
Delivering silver nanoparticles in in vivo studies to test animals via the feed resulted in silver being detectable in the blood and in different organs (liver, lung, kidney, stomach, testicles and brain) even after longer periods of time. After uptake within the gastrointestinal tract the silver nanoparticles are transported throughout the body with the help of the blood circulation and accumulate in the above mentioned target organs. The majority of the nano silver, however, is still excreted in the faeces. From these and other studies it has become apparent that it is mostly the ionic form of silver that is bioavailable for the body. In some cases, an accompanying inflammatory response but no acute toxicity has been detected .
In vitro studies in the laboratory, using different cell lines, have shown that silver nanoparticles are taken up into cells of the gastrointestinal tract either in the particulate or ionic form and are even able to pass the intestinal barrier. The induced cellular stress responses are most likely caused by the released silver ions .

Food packaging with vegetable content. © PhotoSG / fotolia.de
Plastic packaging materials available in the USA and in Asia may contain silver particles that could transfer into the food and consequently be taken up into the body. Studies have proven that predominantly silver ions but also up to 12 % of silver nanoparticles are being released from such packaging materials. However, the detected amounts of nano silver were well below the natural silver load or even below the limit values of 0.05 mg/L silver in water and 0.05 mg/kg of silver in foods as suggested by the European Food Safety Authority (EFSA). Nevertheless the US Environmental Protection Agency (EPA) banned the sale of the investigated plastic containers from this study in March 2014 .
In the EU and in Germany silver is not approved to be used in such antimicrobial packaging materials or as dietary supplement. However, when applied for the decoration of confectionery silver has to be labelled appropriately on the list of ingredients as food colouring agent (E174). Since December 2014 all nanoparticles contained in food and food packaging materials must be labelled with the term "nano" as indicated on the list of ingredients.
Further Information
- Agency for toxic substances and disease registry (ASTDR). ToxFAQs™ for Silver. (Last Access 12/2014)
- Agency for toxic substances and disease registry (ASTDR). Toxicological Profile for Silver, CAS#: 7440-22-4 (Last Access 12/2014)
- European Food Safety Authority (EFSA) (Oct 2014). Topic "Nanotechnology". (last access: Dec 2014)
- Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) (2014). EC-Report: "Are silver nanoparticles safe? Implications for health, the environment and microbial resistance."
- Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) (2014). Opinion on Nanosilver: safety, health and environmental effects and role in antimicrobial resistance, adopted on 11. June 2014. ISBN: 978-92-79-30132-2, DOI: 10.2772/76851
Silver is known to be toxic for bacteria and aquatic organisms this being the reason why it is one of the most studied nanomaterials. Although most studies compare the effects of silver ions (originating form silver salts) with those of silver nanoparticles, it is difficult to distinguish between the effects of the particles and the dissolved ions. The question has not yet fully been answered whether nanoparticles or silver ions are more toxic to environment organisms.
The specific effect of silver nanoparticles on bacteria is lower than expected since the inhibitory effect is caused by released silver ions. By controlling the ion release (for example, by varying the coating or particle size), the strength of growth inhibition can be controlled. Silver nanoparticles attach themselves to the bacterial cell wall and cause damage, which ultimately leads to the death of the bacteria. The type of surface modification affects the binding of the nanoparticles to the bacteria. Similarly, organic materials (such as those found in the environment) bind to the particles and subsequently alter their effect on bacteria [1-6].
Biofilms consist of bacteria and other organisms, which are embedded in a gel-like substance and cover e.g. stones in rivers. The gel-like substance protects the organisms from silver nanoparticles, but not from silver ions. Humic acids, which are found in different proportions in natural waters, on the one hand bind silver ions and reduce the toxicity of particle suspensions. On the other hand, they also stabilise the particles, thereby facilitating the penetration of individual particles into the biofilm. Although the uptake of the silver nanoparticles into the biofilm is increased, the silver toxicity is reduced [8,9].
 With regard to algae, it has not been finally clarified whether nanosilver or silver ions exert a stronger toxic effect. For green algae, silver is known to be toxic, as they are very sensitive to silver and quickly absorb the silver ions. Experiments with green algae showed no differences with respect to the toxic effect of silver nanoparticles and silver ions. This behavior has been attributed to ion liberation from the particles. In addition, the coating has a decisive influence on the toxic outcome [10-14].
With regard to algae, it has not been finally clarified whether nanosilver or silver ions exert a stronger toxic effect. For green algae, silver is known to be toxic, as they are very sensitive to silver and quickly absorb the silver ions. Experiments with green algae showed no differences with respect to the toxic effect of silver nanoparticles and silver ions. This behavior has been attributed to ion liberation from the particles. In addition, the coating has a decisive influence on the toxic outcome [10-14].
Silver in general disrupts the salt metabolism of water fleas affecting not only the behaviour but also the movement of the animals. Whether the silver nanoparticles or silver ions are more toxic to water fleas, is not yet clear due to conflicting results. Size and shape had no influence on the toxicity of silver nanoparticles, even though the animals ingested more small nanoparticles compared to larger particles. In contrast, the type of surface coating significantly affected the effect and uptake of nano silver. In response to nano silver and silver ions, different genes of the water fleas were affected, which probably reflects the differences in the uptake of two substances [10-13,15-20,23].
Whether the silver nanoparticles or silver ions are more toxic to water fleas, is not yet clear due to conflicting results. Size and shape had no influence on the toxicity of silver nanoparticles, even though the animals ingested more small nanoparticles compared to larger particles. In contrast, the type of surface coating significantly affected the effect and uptake of nano silver. In response to nano silver and silver ions, different genes of the water fleas were affected, which probably reflects the differences in the uptake of two substances [10-13,15-20,23].
To simulate realistic environmental conditions, laboratory experiments with silver nanoparticles were conducted in the presence of natural waters ingredients, resulting in a reduced silver toxicity. It is believed that the silver nanoparticles release less silver ions under natural conditions [21,22].
In earthworms, nano silver and silver ions exerted the same toxicity.  Both silver ions and silver nanoparticles show a very limited uptake behaviour by earthworms form soil and are quickly excreted afterwards. The worms show an avoidance behaviour and preferably stay in soils without silver nanoparticles, when exposed to soil with and without nanoparticles. Nematodes show a retarded growth on silver nanoparticles, which can be attributed in part to the silver ions. However, very high concentrations were used, which are not environmentally relevant [24-28].
Both silver ions and silver nanoparticles show a very limited uptake behaviour by earthworms form soil and are quickly excreted afterwards. The worms show an avoidance behaviour and preferably stay in soils without silver nanoparticles, when exposed to soil with and without nanoparticles. Nematodes show a retarded growth on silver nanoparticles, which can be attributed in part to the silver ions. However, very high concentrations were used, which are not environmentally relevant [24-28].

Silver nanoparticles and silver ions are toxic and genotoxic for mussel cells and the ions cause stronger effects compared to the nanoparticles [29].
 Silver nanoparticles exert concentration-dependent toxic and genotoxic effects to fish and cultured fish cells. Nano silver taken up via the water accumulates in gills, intestines and liver and was shown to cause lesions in the liver. Silver nanoparticles present in tank water trigger stress response and damage the gills thereby impairing oxygen uptake. Silver ions and silver nanoparticles show different mechanisms of action in fish. Equally the particle shape (e.g., spherical, rod-shaped) influences the toxic effect, which can be traced back to the number of so-called surface defects. The more often these defects occur in the surface structure of the particles, the more toxic they act. This also explains the difference in effect strengths of various coated nanoparticles [10,12,13,30-32,36-39].
Silver nanoparticles exert concentration-dependent toxic and genotoxic effects to fish and cultured fish cells. Nano silver taken up via the water accumulates in gills, intestines and liver and was shown to cause lesions in the liver. Silver nanoparticles present in tank water trigger stress response and damage the gills thereby impairing oxygen uptake. Silver ions and silver nanoparticles show different mechanisms of action in fish. Equally the particle shape (e.g., spherical, rod-shaped) influences the toxic effect, which can be traced back to the number of so-called surface defects. The more often these defects occur in the surface structure of the particles, the more toxic they act. This also explains the difference in effect strengths of various coated nanoparticles [10,12,13,30-32,36-39].
Silver nanoparticles can attach to the shell of fish eggs with the number of particles depending on the salinity of the surrounding water. In seawater, less silver nanoparticles bind to the eggs compared to freshwater. Nano silver exposure results in a delayed development of fish embryos, malformations of the skeleton and organs, as well as a slower heartbeat [34,35].
For a "realistic" estimate, silver concentrations from laboratory experiments (with various organisms) were set in the context of actually occurring environmental concentrations. Based on these data, the silver concentrations in two Taiwanese rivers were predicted. These calculated values were found to be far below the concentration range identified as critical for fish in the laboratory experiments. From the current point of view, silver nanoparticles present no real risk for environmental organisms [40].
 A comparison of 11 plant species revealed different sensitivities for silver nanoparticles and silver ions, as well as for the various surface coatings of nano silver. In young grass plants the silver nanoparticles, but not the ions caused a delayed root growth. This effect was greater, the smaller the particles were. In a long-term experiment, a community of plants and bacteria was fertilised with nano silver-containing sludge. Biomass was not affected by the nanoparticle treatment and only one of the plant species displayed reduced growth in presence of nanoparticle -containing sludge. Similar effects were observed in communities treated with silver ions [41,42,7].
A comparison of 11 plant species revealed different sensitivities for silver nanoparticles and silver ions, as well as for the various surface coatings of nano silver. In young grass plants the silver nanoparticles, but not the ions caused a delayed root growth. This effect was greater, the smaller the particles were. In a long-term experiment, a community of plants and bacteria was fertilised with nano silver-containing sludge. Biomass was not affected by the nanoparticle treatment and only one of the plant species displayed reduced growth in presence of nanoparticle -containing sludge. Similar effects were observed in communities treated with silver ions [41,42,7].
The sensitivity of various organisms to silver nanoparticles is different. Filter feeders (e.g. water fleas, mussels) were found to be more sensitive than fish. For some of the studied organisms, silver nanoparticles and silver ions had different mechanisms of action. That could be an explanation for the observed differences in the effects of the two silver forms.
Literature
- Xiu, ZM et al. (2012), Nano Lett, 12(8): 4271-4275.
- Radniecki, TS et al. (2011), Chemosphere, 85(1): 43-49.
- Garcia, A et al. (2012), J Hazard Mater, 199-200 64-72.
- Sondi, I et al. (2004), J Colloid Interface Sci, 275(1): 177-182.
- Wigginton, NS et al. (2010), Environ Sci Technol, 44(6): 2163-2168.
- Fabrega, J et al. (2009), Environ Sci Technol, 43(19): 7285-7290.
- Colman, BP et al. (2013), PLoS One, 8(2): e57189.
- Fabrega, J et al. (2009), Environ Sci Technol, 43(23): 9004-9009.
- Wirth, SM et al. (2012), Environ Sci Technol, 46(22): 12687-12696.
- Ribeiro, F et al. (2014), Sci Total Environ, 466 232-241.
- Macken, A et al. (2012), Ecotoxicol Environ Saf, 86 101-110.
- Wang, Z et al. (2012), Environ Toxicol Chem, 31(10): 2408-2413.
- Griffitt, RJ et al. (2008), Environ Toxicol Chem, 27(9): 1972-1978.
- Navarro, E et al. (2008), Environ Sci Technol, 42(23): 8959-8964.
- Zhao, CM et al. (2013), Environ Toxicol Chem, 32(4): 913-919.
- Kim, J et al. (2011), Nanotoxicology, 5(2): 208-214.
- Li, T et al. (2010), Anal Bioanal Chem, 398(2): 689-700.
- Zhao, CM et al. (2012), Environ Sci Technol, 46(20): 11345-11351.
- Zhao, CM et al. (2012), Nanotoxicology, 6(4): 361-370.
- Kennedy, AJ et al. (2012), Environ Sci Technol, 46(19): 10772-10780.
- Newton, KM et al. (2013), Environ Toxicol Chem, 32(10): 2356-2364.
- Lee, YJ et al. (2012), Environ Toxicol Chem, 31(1): 155-159.
- Poynton, HC et al. (2012), Environ Sci Technol, 46(11): 6288-6296.
- Tsyusko, OV et al. (2012), Environ Pollut, 171 249-255.
- Coutris, C et al. (2012), Nanotoxicology, 6(2): 186-195.
- Shoults-Wilson, WA et al. (2011), Ecotoxicology, 20(2): 385-396.
- Hayashi, Y et al. (2012), Environ Sci Technol, 46(7): 4166-4173.
- Meyer, JN et al. (2010), Aquat Toxicol, 100(2): 140-150.
- Gomes, T et al. (2013), Mar Environ Res, 84(0): 51-59.
- Wise, JP, Sr. et al. (2010), Aquat Toxicol, 97(1): 34-41.
- Scown, TM et al. (2010), Toxicol Sci, 115(2): 521-534.
- Wu, Y et al. (2013), Environ Toxicol Chem, 32(1): 165-173.
- Bilberg, K et al. (2010), Aquat Toxicol, 96(2): 159-165.
- Auffan, M et al. (2014), Nanotoxicology, 8 Suppl 1(0): 167-176.
- Wu, Y et al. (2010), Aquat Toxicol, 100(2): 160-167.
- Pham, CH et al. (2012), Ecotoxicol Environ Saf, 78(0): 239-245.
- Chae, YJ et al. (2009), Aquat Toxicol, 94(4): 320-327.
- Griffitt, RJ et al. (2009), Toxicol Sci, 107(2): 404-415.
- George, S et al. (2012), ACS Nano, 6(5): 3745-3759.
- Chio, CP et al. (2012), Sci Total Environ, 420(0): 111-118.
- Yin, L et al. (2012), PLoS One, 7(10): e47674.
- Yin, L et al. (2011), Environ Sci Technol, 45(6): 2360-2367.
If silver nanoparticles are uptaken into the body, they can distribute throughout the body - either as nanoparticles or ions - and accumulate in the organs after overcoming the body's barriers.
Behaviour inside the Body

Possible entry routes of silver nanoparticles into the human body. © Used and adapted with permission from Eckhardt S., Brunetto P.S., Gagnon J., Priebe M., Giese B., Fromm K.M. (2013). Chem Rev, 113(7): 4708-4754. Copyright © 2013 American Chemical Society
The most important entry pathways for silver nanoparticles into the body are via the lungs or via the gastrointestinal tract. Silver nanoparticles have shown to mainly accumulate in different organs such as the liver and kidney but also in spleen, testis and brain. In some cases, gender-related differences in the distribution and retention time of the silver nanoparticles in different organs has been be observed. Female test animals had an increased incorporation of silver in the kidneys whereas the male counterparts displayed a persistent accumulation of silver in the brain and testes. The reasons behind these observations are yet unknown. In general, the distribution pattern of the silver nanoparticles inside the body depends on various factors such as dose, particle size and duration of exposure which also affects the potential toxic effects .
So far the immune system has only been shown to be impacted by silver nanoparticles when using extremely high concentrations, far beyond any realistic exposure. Furthermore there is no concrete evidence that nano silver can cause allergies .
Regardless of the different exposure routes, silver nanoparticles can undergo various biochemical transformations once inside the body for which a variations in particle coating plays an important role. For example, in the stomach the silver nanoparticles dissolve faster due to the acidic environment resulting in an increased release of silver ions. Since silver ions are very reactive they usually agglomerate immediately upon release or interact with and bind to other chemical compounds meaning they no longer exist as ions or nanoparticles. To that effect the silver-ion-complexes (also called organo-silver-complexes) rather than the original silver nanoparticles are taken up and distributed throughout the body. Altering the surface of the silver nanoparticles by means of different coatings allows for a selective adjustment of the ion-release behaviour of the silver nanoparticles .
Up to now there is no evidence that the body is able to metabolise silver ions. With the help of the various detoxification mechanisms within humans, the silver ions are primarily inactivated by binding to other chemical compounds or proteins and then incorporated into the tissues as non-toxic silver complexes (e.g. as silver sulphide). Excretion of silver nanoparticles usually occurs via faeces and urine but very few data are available for this .
Behaviour at the Blood-Brain Barrier
Based on current scientific information it is not yet possible to make a final statement as to whether silver nanoparticles are actually transported across the blood-brain barrier or only deposited in the near-brain regions. Studies dealing with the neurotoxic effects of silver in general (not explicitly with nano silver) could detect silver in the region of blood-brain barrier but no accompanying damage.
Parallel conducted in vitro studies confirmed that the observed neurotoxic effects occurred due to the released silver ions. In addition, the silver nanoparticles triggered an inflammatory response in primary endothelial brain cells resulting in an increased permeability of the cell layer. This mode of action could the enable the transport of normally undesirable substances into the brain .
Behaviour of Uptake in somatic cells
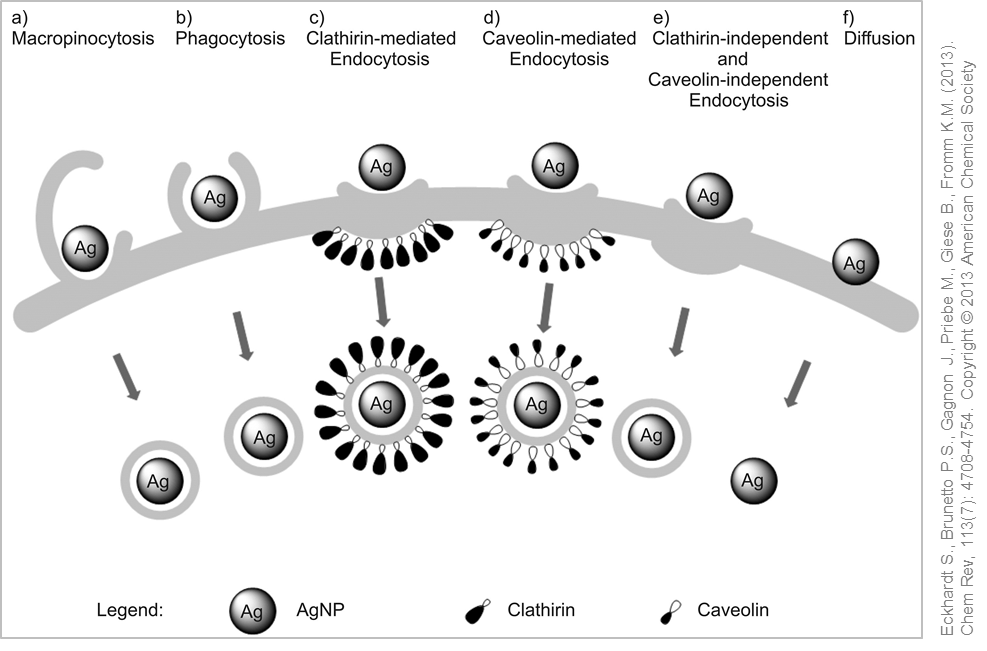
Possible uptake mechanisms for silver nanoparticles. © Used with permission from Eckhardt S., Brunetto P.S., Gagnon J., Priebe M., Giese B., Fromm K.M. (2013). Chem Rev, 113(7): 4708-4754. Copyright © 2013 American Chemical Society
Uptake of silver nanoparticles in the cells has been proved within the framework of in vitro studies. (Nano)particles or small agglomerates are usually taken up via phagocytosis or pinocytosis and can be detected in intracellular vesicles. In phagocytes receptors, so-called scavenger receptors, mediate the cellular uptake processes of the nanoparticles.
Using techniques such as electron microscopy has enabled the detection of silver nanoparticles in membrane-enclosed structures (vesicles) after the uptake into the cell. Due to the acidic pH within the vesicles, the silver nanoparticles are dissolved and silver ions released into the cytoplasm, which in turn can initiate the generation of oxidative stress, inflammation and toxicity. Researchers have described this mode of action as a "Trojan Horse" mechanism .
Silver nanoparticles are not very stable under environmental conditions and are modified due to the influence of various factors (e.g. aging). An important factor is the solubility of the particles that release silver ions in dependence of ambient conditions.
An essential characteristic for estimating the environmental behaviour of nanomaterials is their stability under environmental conditions. Simplified, it is assumed that a high stability leads to a high accumulation in the environment and to better transport properties. Silver nanoparticles are considered an unstable nanomaterial. In an aqueous environment, they tend to dissolve or react with other substances present in the environment. Here, the interaction of environmental factors (e.g. pH) with the particle properties (e.g. surface modification) decides on whether and how quickly certain processes (e.g. sedimentation) occur. Dissolved organic carbon, for example, affects the solubility of silver and thus both the behaviour of the particles and their effects on environmental organisms. Similarly, the type of surface modification of nano silver alters the behaviour with respect to solubility and agglomeration .
Release from facade paint
The release of silver nanoparticles from facade paint was investigated by simulating typical environmental conditions (rain, UV light). After one year, about 30 % of the silver contained in the original paint was washed out. The released nanoparticles were not present individually, but firmly embedded in remnants of the paint matrix (see cross-cutting issues – nanoparticles in paints) .
Behaviour in waste water treatment plants / wastewater
Sewage treatment plants, which purify polluted wastewater from households or industry, are a potential source for nanomaterials being released into the environment. This can occur either via purified wastewater or by application of sewage sludge as fertiliser on fields (as done in some regions). First and foremost, the release of nano silver depends on the incorporation into the respective product (see cross-cutting issues - nanoparticles in textiles).
For silver-containing textiles, it was shown as part of the UMSICHT project that some products released almost no silver, whereas others released high silver amounts into the sewage. Released nanosilver was effectively transported through the sewers to the treatment plant. So far, the concern on a disruption of bacterial treatment of wastewater by silver nanoparticles has not been confirmed. Furthermore, silver nanoparticles are effectively separated from the wastewater and subjected to dissolution- and modification processes. The majority of the particles will adsorb to solids and thus ends up in sewage sludge (see cross-cutting issues - nanoparticles in wastewater treatment plants) .

Section of grass and soil. © andreusK / fotolia.com
Behaviour in soils
Assessing the behaviour of nanomaterials in soils is a challenging task because appropriate methods are still missing. Both the nature of the silver nanoparticles as well as the composition of the soil can vary greatly, which likewise affects the interaction between soil and particles. For example, by comparing 16 natural soils the distribution and solubility of nano silver varied greatly depending on the soil type. The distribution of nano silver in soil differs significantly from the distribution of both of the dissolved silver as well as coarser silver particles .
Silver nanoparticles are considered as unstable under environmental conditions. A variety of processes leads to the dissolution or to modifications of the particle surface. In this respect, both the environmental conditions as well as the particle characteristics determine whether and how quickly these processes occur.
 >
>